|
Eugene Dakin Ph.D., P.Chem Professional Chemist
|
|
|
|
|
Oilfield Basics This section of the website has been created to assist non-technical visitors with general information about producing oil and gas. Areas include how this is its purpose, where does it come from, and why certain aspects are required.
What is the purpose of Oil and Gas?
Oil and natural gas are chemical fuels that help us with common aspects of our life. They allow us to go short and long distances in vehicles, keep us cool in the summer and warm in the winter to name a few items. Many chemical products are manufactured from these raw chemical ingredients such as plastics, pharmaceuticals, cleaners, materials for clothes, and many other articles that are used everyday.
With the world population expected to grow 1.6 billion in the next 15 years or so (circa 2020), places such as China are growing at incredible rates. With automobiles alone, there is a 20% growth of vehicle sales per year.
Natural gas is a cleaner fuel which has lowered the level of smog in major cities. During the mid-century when most homes and factories used coal, smog and health problems were reaching all time highs. During the mid-century, natural gas was also called 'nuisance-gas' because is was largely unwanted, and was usually burned in the field or vented straight to the atmosphere. With an increased demand and price for energy, natural gas was sent to major cities by pipelines and hooked up to houses which also lowered smog and local health problems related to the urban air. Currently, liquid natural gas (LNG) is so versatile that it is placed in a compact liquid form that can be used to fill up transportation vehicles.
Oil is available in many forms, including gasoline (or petrol), Diesel, motor oil, grease, and much more. With the many recycling programs available, old motor oil and grease can be chemically changed to gasoline or diesel or a variety of other chemical products.
Where does Oil and Gas come from?
Natural gas and oil are created from the natural biological and chemical degradation of prehistoric plants and animals. When the remains of these life forms settled to the bottom of seas, they were covered with various material such as rocks and sand. Over many years, these remains were continuously covered by many layers of rocks and sand which left them deep in the earth. The increased pressures and temperatures that exist in the earth chemically changed this material into petroleum. The petroleum contained in the pores of this rock is commonly called source rock. The trapped oil in source rock is referred to as reservoirs. Crude oil usually contains various concentrations of natural gas, oil and water.
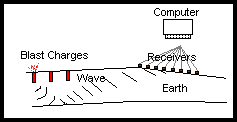
How do you find Petroleum?
When people ask about Chemists, or Chemistry, they often think about their pharmacist or of producing medicinal drugs that Medical Doctors give them a prescription for. Although this is one of the many fields in which Chemists are involved, we are involved in over 95% (directly or indirectly) of the materials, methods, and products used to help, aid, and assist all aspects of oil and gas production. When looking at a wellsite, the oil and gas themselves are chemicals, along with the pipe (made of metals which are chemicals), valves (again made of metals), have corrosion inhibitors (more chemicals), and the employees themselves wear Personal Protective Equipment (plastic ear protection, fire resistant clothing made of chemicals), drive to a wellsite in a vehicle half made of plastic chemicals, and metal chemicals.
It is safe to assume, that the everyday items which are manufactured and used in everyday life are chemicals. When chemists make new chemicals, and/or increase the level of chemical technology, the entire world is influenced. What Other Professions does the Chemist Work With?
Biologists - With the ever increasing importance of chemical molecules such as: Deoxyribosenucleic acid (DNA), Ribosenucleic acid (RNA), hormones, enzymes, cells, membranes, nutrients, vitamins, and more.
Geologists and Geophysicists - Chemicals compose of all the matter that these professionals study. Different types of rock exist which are all made up of, and studied from the laws of chemistry.
Engineers - Everything that has any aspect of engineering is directly related to chemistry, from the matter of the which makes up metals, wood, plastic, and other physical material is all created from and by chemists. This can be in the form of electronic wires, cement, building materials, piping, petroleum, and more.
Forestry Professionals - All living organisms (from bugs, to trees) which make up everything that touches a Forestry Professional deals directly, or indirectly with the Chemist.
Environmentalists - When the environment needs help in one way, shape, or form, the environmentalists come to the rescue with a team of chemists to help them out with their tasks. The chemists determine what a system currently has, and has many methods of treating and helping environmentalists to assist mother nature.
Government - When the earth, air or water are involved, governments rely heavily on chemists to make their economies soar, which increases taxation, budgets, and helps the entire country.
Other professionals - If it is physically created, modified, or viewed, chemistry has been involved. From the ultra high-tech planes which defend countries, to the food we eat, and the glasses we drink from, every aspect has chemists and chemistry involved.
All forms of matter are created from various combinations of elements from the Chemists Periodic Table of Elements.
What are Various Titles of Chemists?
As technology increases and forms more specialists, more titles are recorded. Here are a few of the titles that immediately come to mind:
Inorganic Chemistry - This extremely wide area of chemistry deals with all matter of an inorganic nature. For example, iron, carbon, salts, and much more
Organic Chemistry - As a general rule of thumb, organic chemists deal with Carbon, Oxygen, and Hydrogen elements. This vast area of chemistry deals with pharmaceuticals, living matter, and much more.
Physical Chemistry - Any material that interacts with another form of matter and the expression of laws and forces of nature are largely of this category.
Nuclear Chemistry - This seems to be self explanatory. Any energy radioactivity, or nuclear medicine has chemists widely involved in this area. This could be a nuclear reactor, to radiation treatment for cancer patients.
Metallurgical Chemistry - Although this area combines areas of the aforementioned disciplines, it is largely focuses on metals and their many combinations.
Industrial Chemistry - Creation, modification, or treatment of any industrial product involving the previous categories on a large scale.
Electronic/Electrical Chemistry - This fascinating area of chemistry has a focus on molecules interacting with each other from an electrical/magnetic perspective. From new computer chips, to anodic and cathodic corrosion protection and batteries. Solar Cells which are an alternate source of energy are a good example of the benefits of Electronic Chemistry.
Water/Wastewater Chemistry - When you turn on the tap for a fresh glass of water, or have consumer water (from your dishwasher) placed down the drain, a chemist must ensure that it meets environmental regulations, and treats the water so that it meets the specifications for discharge. This is the basis of the American Water Works Association (AWWA).
Quality Control/Assurance Chemistry - Once chemicals have been created to a suitable form, chemists are involved to ensure that these materials meet certain specifications or guidelines. This is a necessary part of ISO 9000 certification.
Corrosion Chemistry - Within industrial chemistry, is the protection and treatment of materials. There are almost more chemists involved in the North American Corrosion Engineers (NACE) than other professionals.
Environmental Chemistry - New regulations have passed which further widen the scope of chemistry. When dealing with site reclamation, Environmental chemists are involved.
Process Chemistry - In manufacturing matter, most business's have a process chemist which guides and monitors chemical processes .
Agriculture Chemistry - Modified strains and nutrients provided to farmers are created from and by Agriculture Chemists. This could be from the manufacturing of pesticides, application of pesticides, to nutrient and growth monitoring/stimulation of all forms of life in the wide field of agriculture.
Pyro/Demolition Chemistry - This widely respected field is used in work (mining rocks) and enjoyment (fireworks and firecrackers), and war (firearms, munitions, etc).
Polymer Chemistry - This very wide area of chemistry involves the manufacturing/extrusion/creation of polymers or plastics.
Medicinal/Pharmaceutical Chemistry - When people take medication from a medical doctor, the interactions between conflicting medications must be monitored and determined.
Analytical Chemist - Process chemicals and materials need to be monitored for performance. It is the analytical chemist who can separate molecules into its basic building blocks and provide the information to people.
Instrument Chemist - The equipment that aids and assists all chemists eventually needs repair. Instrument chemists provide this technical service.
Sales Chemist - Where would business's be without the sales chemist? Wherever chemicals are being sold, you will find a sales chemist to provide help with your unique needs.
Lubricant Chemist - The next time that you get an oil change, the thousands of hours required for research and development of the lubrication products are created by these hardworking chemists.
Fuel Chemists - With the wide expanse of hydrocarbons and any nations increased need for energy, these chemists are available to create and monitor needed fuels for automobiles, transport ships, airplanes, heating oil, and more.
Waste Management/Recycling Chemists - When matter is to be reused, (such as recycling glass or plastic) the chemist determines the working parameters, efficiency, and material balance of the systems.
Computer Programming Chemistry - Any chemical simulation program which is created by a Computer Programming chemist.
Contact Eugene for more information.
|
|
| © 2006 - Eugene Dakin -
|